The noble art of tick defense
Ticks are notorious for spreading Lyme disease. Erin I. Garcia de Jesus talks with biologists trying to find new ways to manage tick-borne diseases. Illustrated by Rob Soto and Anisha Parekh.

Illustration: Rob Soto
As Fauna Yarza opens the door of what looks like a refrigerator in a lab, there is no blast of cold air. Instead, five glass containers that could be mistaken for cookie jars sit inside at room temperature. And in place of chocolate chip cookies, each jar holds clear tubes that sport orange caps and are stuffed with a thick piece of white paper. The papers are speckled with mobile black spots: ticks.
Many of the eight-legged creatures cluster in groups, which look like a dark stain from far away, but a few sit alone in a sea of white. Some are large adults; other tubes house smaller and younger individuals – nymphs and larvae.
“If I pull the cap off, they’d all come running at you,” says Yarza, a graduate student at UC San Francisco, as she inspects a tube filled with adult ticks.
The incubator houses ticks that carry the cause of Lyme disease, a corkscrew-shaped bacterium named Borrelia burgdorferi. When ticks infected with this bacterium bite and attach themselves to a host animal – sometimes for several days as they feed slowly on blood – the bacteria can invade the unsuspecting target.
Tick bites transmit a variety of different diseases across the U.S. and the world. Studying them will help scientists such as Yarza and her advisor, Seemay Chou, learn how ticks control the microbes they pass on to animals and humans. Chou wants to know how the tick’s immune system responds when it picks up B. burgdorferi from a small mammal – and whether there are unknown pathogens hiding in tick bodies. Understanding how ticks control the microbes they transmit – as well as the ones they don’t – could help researchers get a better handle on how to manage tick-borne diseases.
“Lessons learned in ticks could help us think of new ways to try and block transmission of Lyme disease,” says Seemay Chou, an assistant professor at UC San Francisco.
Breaking the cycle
In the mid-1970s, a mysterious outbreak of arthritis hit a group of children and adults living in Lyme, Connecticut and its surrounding areas. Researchers eventually determined that ticks were the source – and in 1982 they singled out the curly spirochete bacterium, Borrelia burgdorferi, as the cause of what they called Lyme disease.
Today, Lyme disease exists in the U.S. as well as parts of Europe and Asia, where it is called borreliosis. And it is on the rise in the U.S. According to the U.S. Centers for Disease Control and Prevention, diseases transmitted by ticks more than doubled from 2004 – 2016; Lyme disease accounted for 82 percent of these cases. Reasons for the spike are unclear, but possibilities include increased awareness in clinics, climate change and human encroachment into tick habitat.
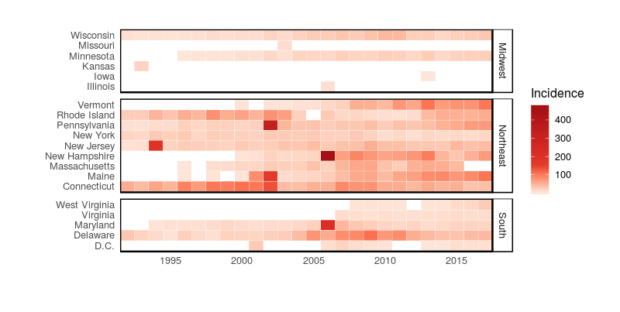
Lyme disease is on the rise in the U.S., according to the CDC. Since the 1990s, it has spread to multiple states in the Midwest and South. Image by Erin I. Garcia de Jesus
In the U.S., Lyme disease is most common in the northeast, mid-Atlantic region and the Midwest. There, the blacklegged tick – Ixodes scapularis, a species typically found in wooded areas – transmits B. burgdorferi. And Lyme disease shadows the tick’s life cycle. Ticks bite small mammals, such as white-footed mice, and feed on their blood to grow from minuscule larvae to pinhead-sized nymphs to apple seed-sized adults. Each time it bites a new host animal, the tick can pick up the bacteria that cause Lyme or pass them on to a new host.
Lyme disease is not restricted to the eastern U.S., however. In 1985, ten years after the outbreak in Connecticut, researchers reported that they’d found ticks with Borrelia on the West Coast. Even 30 years later, fewer people in the western U.S. know that Lyme exists in the region.
One of the reasons is that the disease remains “fairly rare” in the West compared to the East, says Richard Ostfeld, a disease ecologist at the Cary Institute of Ecosystem Studies in New York. And the Lyme disease cycle is slightly different in the East and West. Western Lyme is spread by the western blacklegged tick – Ixodes pacificus – which feeds on mice, western grey squirrels and other animals.
Overall, Lyme disease is challenging to control. Myriad strains of Borrelia bacteria circulate in the U.S. And each strain could produce marginally different symptoms. Some researchers, such as Ostfeld, are developing methods to control ticks, including treating white-footed mice with the active ingredient of the pesticide Frontline – used to remove ticks from dogs and cats – to kill attached ticks.
Others, such as Chou, are turning to the inner workings of the tick. Perhaps, Chou says, if we understand how ticks respond to microbes in general – especially the pathogens they carry – researchers could identify new targets to break the Lyme disease cycle.
New frontiers
Chou began her scientific career as a structural biologist. She studied how proteins in bacteria and viruses folded together, hoping to learn how they worked. While working at the University of Washington in Seattle, however, she made an odd discovery.
Chou was studying the structure of a toxin that some bacteria use to kill competitors. She had already described the structure of a toxin from one bacterium and wanted to explore similar toxins from other species. She and her colleagues started looking for other examples, and they were surprised to find similar proteins in Ixodes ticks.
Chou wondered whether the ticks were using these toxin-like proteins to manage infections. She spent two years learning the ins and outs of tick biology: rearing ticks in the lab and learning how to work with existing research tools. She and her team discovered that the toxin-like proteins in ticks originally came from bacteria hundreds of millions of years ago. Over time, the toxins became part of the tick’s defense system against invading bacteria – and could even slow the growth of Lyme-causing bacteria in the blacklegged tick.
From then on, Chou was a tick biologist. “I was totally hooked,” she says.
Chou’s finding, published in the journal Nature in 2015, was an intriguing example of genes moving between bacteria and an animal. Typically, parents pass their genes – which then make proteins – to their offspring. Biologists describe this type of genetic sharing between generations of the same species as “vertical” gene transfer. But in this case, the gene transfer had crossed into a new species – from bacteria to ticks. It’s an example of “horizontal” gene transfer across species.
It’s like when one language adopts a word from another. The foreign word uses similar letters, but they are arranged in a different order and for a while the word doesn’t make sense in casual conversation. But over time, it could be incorporated into that language.
The tick had co-opted genes from bacteria to defend against infection. What other genetic tools do ticks have to defend themselves?
It was a big question; scientists don’t fully understand tick immune systems. That’s partly because the tick’s full genetic library – or its genome – was not available until 2016. Scientists can use model organisms, such as fruit flies, to help them understand insect immune genetics. But ticks are arachnids, more closely related to spiders than fruit flies. That means that much of the genetics research on insect immunity doesn’t apply to ticks. Myriad unknown genes could lead a tick’s attack against microbial intruders.
Now, Chou has left structural biology behind and focuses her research efforts on studying tick immunity. She has deployed her graduate student, Yarza, to uncover how the immune system responds in ticks that harbor B. burgdorferi. And together, the two hope to learn what defenses Lyme disease bacteria activate in ticks.
Probing ticks
Working with live ticks requires a few precautions. The organisms are small and mobile – easy to lose if they escape from their tubes.
“[Ticks] seem intimidating, but they are actually easily thwarted by tape,” says Yarza.
Back in the lab, Yarza whacks a tube filled with male blacklegged ticks against a countertop to get ticks off its lid. The small black dots fall into a clump at the bottom of the tube. Yarza opens the tube; grabbing a pair of tweezers, she deftly pulls one straggler off the edge of the cap and places him in a circular white container on the countertop. The container’s white background makes him easier to spot, she explains.
Yarza picks the tick up again with her tweezers, holding him to the center of a glass microscope slide. She attaches to a piece of Scotch tape to his abdomen, anchoring him in place so she can cut off one of his forelimbs with a scalpel.
“[Ticks] seem intimidating, but they are actually easily thwarted by tape,” says Yarza.
When a clear liquid comes out of the severed limb, she collects it with a thin tube. The liquid contains the tick’s immune cells, called hemocytes. In the future, Yarza will analyze ticks that are infected with B. burgdorferi and extract their hemocytes’ genetic material. She will not only focus on blacklegged and western blacklegged ticks, but also other tick species that don’t transmit the bacterium to humans. Studying multiple species can help scientists understand why some ticks transmit Lyme disease and others don’t.

Taping ticks to a slide allows scientists to look at them under a microscope without having to worry about its escape. Photo by Erin I. Garcia de Jesus
Immune systems act like an army defending its territory. Hemocytes are like the soldiers in the army, holding weapons – proteins – that can beat back intruders. The tick’s soldiers might attack invading bacteria in diverse ways. Yarza’s job is to figure out what their weapons are. Are the soldiers producing missiles to kill bacteria in a single blow? Or are they sending messages to their fellow warriors to coordinate a group strike?
Chou and Yarza hope to answer that question by studying what genes code for the protein weapons the tick’s hemocytes carry. Some may be similar to the toxin-like proteins that Chou wrote about in Nature or additional proteins discovered by researchers studying ticks. Others will be new. Part of the puzzle will be working out how they fit in the tick’s immune system.
Does the tick immune system help transmit Lyme? Can scientists manipulate it to block bacterial growth or transmission? These are some of the questions Yarza and Chou are working to answer.
“There’s a long way to go,” says Yarza.
Looking inward
While some of Chou’s work centers on how ticks defend themselves from bacteria that they can pass to humans, she and other scientists are interested in bacteria that naturally live in ticks. These natural bacteria – called the microbiome – might determine what pathogens a tick can transmit.
Researchers have discovered that altering the microbiome can interfere with disease transmission in insects. For example, if mosquitoes carry certain bacteria, they can no longer transmit dengue virus. Exploring the tick microbiome could reveal similar relationships – or new ones – that researchers can exploit.
“I think it could be a path forward for understanding how we can manage transmission of Lyme disease and tick-borne diseases,” says Andrea Swei, a disease ecologist at San Francisco State University.
Part of Swei’s microbiome work focuses on western blacklegged ticks. Swei wanted to learn how the tick’s microbiome changes when it feeds on different hosts, such as the western fence lizard.
The lizard is part of the reason Lyme disease is not as prevalent on the West Coast as in the East. The lizard carries something special in its blood: a protein that “cleanses” Borrelia bacteria from ticks. Western blacklegged tick nymphs thirst for lizard blood; when they feed on the lizards, they ingest this cleansing protein, which clears Lyme-causing bacteria from their bodies. The nymphs then mature into adults that lack the Lyme-causing bacteria. This explains why nymphs in California are more likely to carry Lyme spirochetes than adult ticks and could help explain why Lyme is seen in the West at lower rates than the East, says Robert Lane, a medical entomologist at UC Berkeley.
Blacklegged ticks populate the eastern U.S. while western blacklegged ticks inhabit the West Coast. Illustration by Anisha Parekh
A few years ago, Swei wondered whether the lizard’s Lyme-clearing protein alters the tick microbiome in other ways. She collected tick larvae from wild mice and lizards and used DNA sequencing to catalog what bacteria were living in the ticks’ microbiome.
“We found really striking differences in the microbiome complexity” between ticks feeding on lizards and those feeding on mice, says Swei. Her study, published in the International Society for Microbial Ecology Journal in 2017, showed that ticks fed on lizards had fewer bacterial species in their microbiome than those that fed on mammals such as mice. That lower abundance seemed to be helpful for Lyme disease transmission, according to Swei.
But the Lyme bacterium is only one of many microbes found in ticks, which also harbor viruses, parasites and other bacteria. And ticks can transmit more than one pathogen at once, putting people at risk for contracting multiple diseases with each bite.
“I think of the biggest holes [in our knowledge] is the full range of microbes that ticks carry,” says Chou. Much of our information about tick pathogens, she explains, comes from what we know from the blacklegged ticks on the East Coast. On the West Coast, it is still not known how many other microbes can transmit from ticks to humans, she says.
To begin to fill in those gaps, she teamed up with Amy Kistler, infectious diseases group leader at the Chan-Zuckerberg BioHub, a research center that hosts scientists from UC San Francisco, Stanford and UC Berkeley. At the BioHub, Kistler probes mosquitoes for potential pathogens, but now she is turning to ticks. She and Chou hope to discover what microbes – from bacteria to viruses and parasites – western blacklegged ticks carry and whether they cause diseases.
“I have a natural curiosity about insects that land on me and are sucking my blood and mixing what’s in their body into my body,” Kistler says.
The project with Chou has made Kistler more aware of ticks. In early December 2018, after the two had begun working together, Kistler’s dog came back from a walk covered in about 40 western blacklegged ticks. Unfortunately, none of the ticks from her dog made it back to the lab for analysis, but she is determined to understand what pathogens the ticks carry: “Now, I’m very attuned to tick outbreaks,” she says.
If Kistler and Chou are successful, it could help researchers begin to study tick-borne diseases before they become a problem. It will also help researchers understand how often ticks transmit the pathogens that they ingest from other animals to humans.
“At the end of the day, I’m just a biologist that’s very curious,” says Chou. “If you pull on one thread, you have no idea what you’re going to learn next by studying these systems that have been evolving for hundreds of millions of years.”
© 2019 Erin I. Garcia de Jesus / UC Santa Cruz Science Communication Program

Erin I. Garcia de Jesus
Author
B.S. (microbiology) New Mexico State University
Ph.D. (microbiology) University of Washington
Internships: Smithsonian National Museum of Natural History (Washington, D.C.) and American Geophysical Union (Washington, D.C.)
Growing up, I loved to roam my yard collecting insects. I punched holes in the lid of an old plastic ice cream container and stuffed it full of grasshoppers and butterflies. I particularly loved black swallowtails; inspired by their yellow-spotted beauty, I wrote stories featuring elusive, imaginary butterfly species in the Alaskan wilderness.
In college, I saw insects in a different light: as vectors of disease. I studied how mosquito-borne viruses impact human health, but I was happier writing about my work and hearing about other projects than doing my own research.
I eventually realized that as a science writer I could write about anything, from viruses to my beloved butterflies. I grabbed a pen, laid down my pipette and embraced doing what I love most: learning and writing about science.

Rob Soto
Illustrator
B.F.A (illustration) Maryland Institute College of Art
Internship: Alf Museum of Paleontology (Claremont, California)
Rob Soto is a freelance Science Illustrator and Paleoartist. The goal of his work is to inspire curiosity and understanding for the extinct creatures of Earth’s past and endangered animals that share the Earth with humanity. His creative projects focus on species that are often misrepresented or ignored by popular culture. Rob strives to create work that feels realistic and imbued with dynamic energy by crafting thoughtful sketches out of movement and captivating detail.
Happiest in wilderness of his home in New Mexico, with a pencil in one hand and a beer in the other.

Anisha Parekh
Illustrator
B.A. (interdisciplinary studies) Thompson Rivers University
Internship: Pacific Salmon Foundation (Nanaimo, British Columbia)
Anisha Parekh is an artist and writer from Kamloops, British Columbia. She has long held a passion for creating; from making valentines cards as a child, to designing an illustrated children’s book for her undergraduate thesis. Anisha spends her small amounts of free time cooking and baking for her loved ones, thinking about her next vacation, and chasing the mastery of illustration, graphic design, photography, creative writing, and everything in between.
Through art, Anisha can interpret and communicate ideas creatively to help invoke curiosity in others. The world we live in is a captivating place, and above all, art allows Anisha to observe and replicate it while providing insights into the magic of life.
Extremely interesting. My daughter now works with Seemay at the Mission Bay campus. I live in northwest Arkansas but have been in the San Francisco lab a couple of times.