An ongoing hunt for pain’s off-switch
Doctors are searching for a better solution for patients with chronic pain — a way to switch their pain off at the source, reports Nicoletta Lanese. Illustrated by Sylvia Heredia and Brett Bell.
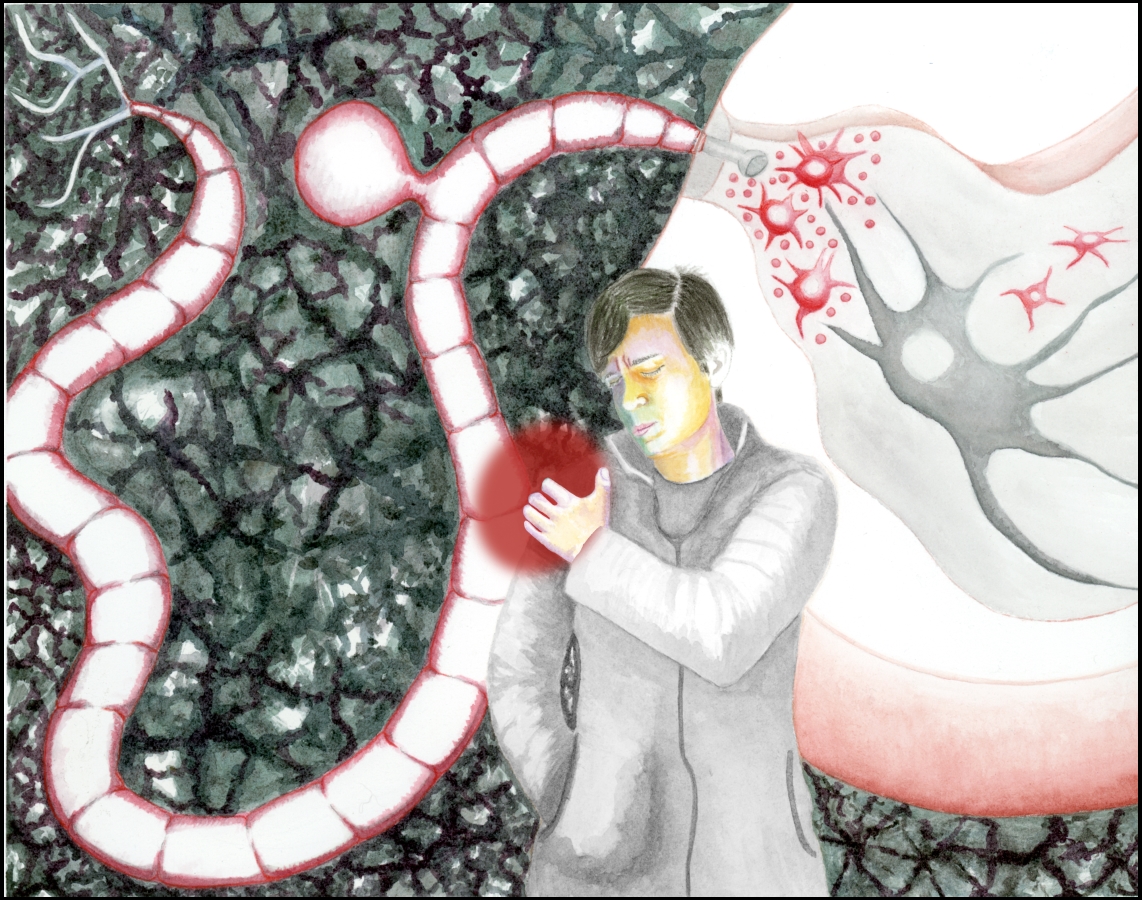
Illustration: Sylvia Heredia
Stephanie Morse remembers being roused in the emergency room one summer night in 2008.
“I took too many pills,” she told the hospital staff. “I don’t know what I took.”
She was kept overnight on a 5150, meaning the doctors thought she may have attempted suicide. But that wasn’t the case, she insists. Morse said she had accidentally overdosed on her prescribed medications for chronic pain, sent to the ER by a combination of gabapentin, Ambien and a small glass of wine. She was nearly a statistic, illustrative of a disturbing trend: Over 6,600 American women died of prescription painkiller overdose in 2010—more than five times as many as in 1999.
“I don’t want to think that I won’t get better.”
Six months before Morse’s overdose, surgeons had removed a spattering of calcium deposits from her right shoulder. A relentless ache had flared up in their place. After seven prescriptions, countless medical appointments and that fateful trip to the ER, her pain was finally diagnosed as Complex Regional Pain Syndrome, or CRPS — a condition in which pain festers in a limb long after an injury, causing swelling, discoloration and changes in sensation.
Physician Vivianne Tawfik diagnosed Morse with complex regional pain syndrome and has treated her at Stanford Hospital for the past nine years. As a pain specialist, Tawfik works to help Morse and her other patients manage their pain. Tawfik’s work is increasingly important: over 8 percent of American adults report being in severe pain every day, and pain medications rank as the second-most dispensed prescription. Overall, an estimated one in three American adults suffer from chronic pain—that which has persisted over three months. Morse’s specific pain syndrome is a relatively rare form of chronic pain, with about 55,000 newly diagnosed cases each year. The pain subsides for some and persists for years in others.
To tackle her own patients’ pain, Tawfik employs a laundry list of therapies, including nerve blockers, antidepressants, anticonvulsants and narcotics. But many of Tawfik’s patients tick straight through that list and remain racked with pain. They tote around packed pillboxes, swallow down their empty promises of freedom from pain, and are left feeling exhausted, foggy and constipated, rather than relieved. The stakes are even higher for women in Morse’s age group, who have the highest risk of dying from a prescription painkiller overdose.
At the heart of the issue is a question that has plagued medicine for many years: Why does some pain dissipate after an injury has healed, while other pain hangs around long after the fact? If pain physicians knew that, they could prevent the onset of chronic pain, rather than trying to numb patients once it takes hold.
Tawfik hopes to someday realize that future. She is studying the transition from normal, short-term pain to chronic pain, using mice as her subjects. Neuroscientists have known that cells called microglia amplify pain signals on their way to the brain. If this boost persists after the painful injury itself has healed, it may lead to chronic pain. If this is the case, Tawfik hopes she might be able to alter the activity of microglia, tune down the incoming pain signals and turn off that prolonged pain.
Tawfik’s challenge will be moving her research from mice to humans. Pain researchers have been under fire—often friendly fire—as some scientists increasingly argue that pain experiments in mice have little relevance in human disease. While pain signals move through mice and humans similarly, it’s not possible to recreate the suite of emotional, psychological and physical aspects of human pain in a rodent. Some researchers have also constructed unrealistic experiments, often using only male mice, for instance, or measuring irrelevant symptoms. That has made their research less helpful to human patients.
“People study drugs in mice and then say, ‘This is going to cure pain!’” Tawfik explained. “But they try to move it forward clinically and it’s a bust.”
Despite a mixed track record, Tawfik says, animal studies are an irreplaceable piece in the pain research jigsaw. For patients like Morse, whose pain persists in defiance of the strongest drugs, these studies offer some hope for relief. It has been 10 years since Morse’s shoulder surgery, and her pain remains a mysterious moving target and a given in her daily existence. It has migrated to her left shoulder, and she compares the sensation to the pounding throb you feel after being hit with a hammer, or the radiating pang that erupts after your dentist nicks a nerve during oral surgery. Her pain is heavy, weighing down her body and mind like an invisible sandbag.
“I keep trying to find ways to be optimistic — that’s the hard part,” Morse said. “I don’t want to think that I won’t get better.”
Tawfik is striving to improve upon pain studies of old, and to achieve real results in a field infamous for its shortcomings. Her largest ongoing mouse study hints at a potential remedy for chronic pain, and she is now racing to see if it might work in humans, too.
Turning up the volume on pain
The seed of Tawfik’s current research took root in the early 1990’s. Before then, scientists assumed that neurons—the excitable messenger cells of the nervous system—were wholly responsible for relaying pain signals through the body. Neurons send electrical signals down an output cable, known as an axon, which releases chemical messages to neighboring cells. Neurons are surrounded by cells that lack axons, called glia. Glia means “glue” in Greek, and glia were once thought to bind neurons together, providing them with insulation and structural support.
“They start spewing out substances that make pain neurons go wild.”
But as technologies were developed to better study glia, scientists found evidence that the cells are more than just brain stucco. Many neuroscientists dismissed the idea at first, but now years of extensive research have provided too much evidence to ignore.
Linda Watkins, a behavioral neuroscientist at the University of Colorado Boulder, was among those pioneering scientists that got glia into today’s textbooks. Her early studies of influenza-related pain helped to define the broader role for glia. “It turns out that all the symptoms of the flu are created by glia,” she said. “And pain is part of that.” When you’re struck with the flu, you want nothing more than to crawl into bed, she said, but to your dismay, even the bed sheets hurt. Though they have no axon or other direct line of communication with the brain or spinal cord, glia appear to contribute to that pain signal.
“You can think of them as turning up the volume on pain,” she said. “If they become activated, they start spewing out substances that make pain neurons go wild”—such as substances called proinflammatory cytokines that call immune cells to assemble at an injury site and ignite inflammation to fight infection. Such chemicals make neurons more sensitive to incoming pain signals, influencing how intense pain feels down the line. After “turning up” pain for a certain length of time, glia can become prone to faster, stronger and longer activation, said Watkins.
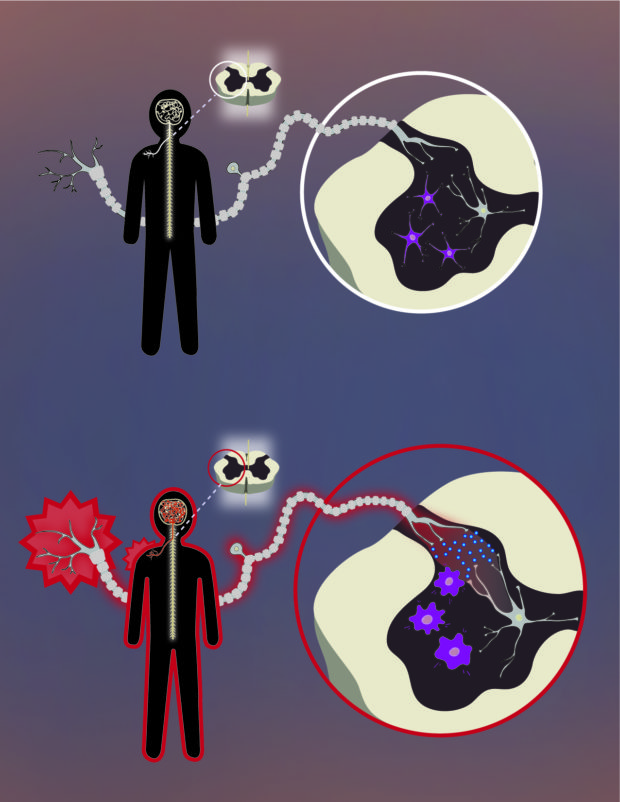
Microglia amplify pain signals in the body and may trigger chronic pain if activated too long. Illustration: Brett Bell
Research suggests this prolonged glial activation might push short-term pain over the edge so that it becomes chronic. But no one knows exactly how. This is the conundrum Tawfik aims to solve. By studying glial cells found only in the brain and spinal cord, called microglia, she hopes to understand how these cells contribute to chronic pain and how to stop it in its tracks.
Making do with mice
Clad in seafoam gloves, a dandelion-yellow smock, sky-blue mask and plastic booties, Tawfik stands out against the white walls of the “mouse room” in a nondescript building on Stanford campus. Here, racks of plastic cages serve as rodent condominiums, each one housing four tenants.

Vivianne Tawfik on Stanford University campus. Photo: Nicoletta Lanese
Tawfik hopes these rodents will help usher in a new era of pain studies — one when long hours in the mouse lab consistently lead to helpful solutions in the clinic.
Pain is a complex interaction of physical, emotional and psychological factors, and scientists have yet to figure out how to ask a mouse how it’s feeling, emotionally. Researchers can only study pain-like behavior and nociception—how the nervous systems reacts to painful stimuli—in animals. For instance, a researcher may prod a mouse’s injured paw and note whether it pulls away and how quickly.
As a result, historically, the pain field has often stumbled to move from animal studies to human treatments, says Jeffrey Mogil, the Canada Research Chair in Genetics of Pain at McGill University in Québec.
“Close enough” may not really be good enough.
“We’re not really mimicking the diseases that are associated with pain in humans,” he says.
In studies of arthritic pain, for example, rather than truly giving a mouse arthritis, researchers will inflame its paw and study that symptom specifically, he said. But pain is more than a collection of individual symptoms, and researchers sometimes lose the big picture by zooming in on just one pixel. A pain researcher himself, Mogil says scientists often settle for animal studies being “close enough” to actual human conditions.
But “close enough” may not really be good enough.
Compounding the problem, the vast majority of pain research has been done in male mice from the same genetic strain. Differences between males and females may be especially relevant in chronic pain, which affects far more women than men.
“There’s serious questions as to whether that strain should be the default, and whether female animals should be basically excluded,” Mogil said. “They may or may not be widely representative of all mice, and may not be relevant to people at all.”
Tawfik has attempted to deal with these concerns by building a better animal study, one that very closely replicates what she sees in clinic. Many of her patients first fracture a bone; then, after the cast comes off, the fracture pain lingers and develops into complex regional pain syndrome. Tawfik recapitulates this scenario in her mouse experiments, studies symptoms she sees in humans, and is careful to represent her most common patients: women.
Manipulating microglia
Back in the Stanford mouse house, Tawfik cups an injured mouse in her gloved hand. Its right hind leg is healing from a fracture and secured in a tiny cast. This animal is one of dozens of mice that Tawfik has bred with disabled or low-functioning microglia.
Using genetic engineering technology, Tawfik is working to disable different genes along the pain pathway. This mouse lacks an essential microglial protein, and thus has no functional microglia. In another experiment, Tawfik disables this component with an injectable drug. She can then investigate how different levels of microglial activation in her mice correspond to the intensity of their pain symptoms.
…the feeling of clothing against their skin can become excruciating.
The classic symptom of CRPS is long-lasting pain that is stronger than it should be given the injury that triggered it. Patients may also have muscle tremors and weakness, brittle nails and slow-growing hair on the affected limb. They may become hypersensitive to painful sensations; a minor cut or bruise might cause severe pain. They may even become sensitive to normally painless sensations, and a light touch or the feeling of clothing against their skin can become excruciating. Morse, for instance, says when she walks on pebbles with bare feet, it sometimes feels as if she’s walking on shards of jagged glass.
Tawfik studies these symptoms in mice by observing whether her genetically altered mice become more sensitive to touch and heat after injury. Tawfik’s patients also develop swelling, redness, pain and warmth in their injured limb, and her mice exhibit the same, she says.
Tawfik boxes up two mice and carries them to the testing room to demonstrate. Inside the room, she gently places the mice atop a small elevated platform made of chicken wire. The animals are confined within clear vertical plastic tubes, to keep them from scurrying.
Tawfik uses weighted filaments to gently poke mice to test their sensitivity to sensation. Taking a 2-gram filament in hand, Tawfik prepares to prod a mouse; this particular subject has no injuries or modifications to its microglia, so it should not be abnormally sensitive to pain.
Tawfik pokes the right hind foot of the mouse through the chicken wire. It doesn’t react, even after 5 seconds of pressure. Tawfik writes this down in a chart, then reaches for a heavier 4-gram filament. With this poke, the mouse pulls back its paw almost immediately. An uninjured mouse will usually respond to pressure between 1.5 and 2 grams. To a human, these pokes feel like the slightest brush of a cat whisker. Injured mice will respond to even lighter filaments, at weights between 0.02 and 0.1 grams, Tawfik says.
“If mice have some sort of injury, they tend to really respond at a very low threshold,” she says.
The same goes for the mice’s sensitivity to heat. To test this, Tawfik places them on a hot plate and slowly raises the temperature. She records a video of each trial so she can watch how their behavior changes as she turns up the heat. She notes when they first pull back their paws or jump away from the plate and then tracks their behavior moment to moment as the heat persists, despite their efforts.
Time and time again, she’s gotten the same results back…
Tawfik also uses an imaging technology called Positron Emission Tomography to scan the brains and spines of her mice. She plans to use the same technology in human patients to take a snapshot of their own nervous systems. Tawfik uses the scanner to measure how active her mice’s microglia are before and after injury. She hopes that the scans will tell her whether injuries cause microglia to become more active in her mice, and how that activation might be paralleled in humans.
Over the last year and a half, Tawfik has run six groups of mice through this set of experiments. Time and time again, she’s gotten the same results back: manipulating microglia, even temporarily or to a moderate degree, can completely change the trajectory of pain. By disabling or deleting just a fraction of a mouse’s microglia—as few as 25 percent—Tawfik can block their abnormally strong pain response before it takes hold. When she allows the mice’s microglia to grow back to a normal level, they’re still fine. It seems Tawfik may be flipping pain’s off-switch.
These are promising results, but the mechanisms that cause microglia to prolong pain remain a mystery. Tawfik wants to solve that question so that other scientists might develop medications to interfere with microglia and perhaps provide a quick fix for chronic pain.
A pain-free tomorrow
“How can I be there for them when I’m not there myself?”
Such a drug could give patients like Morse a new lease on life. From the outside, you’d never guess Morse was living with debilitating pain. Her fashionable outfits, sparkling nail polish and sleek sandy hair seem incongruent with someone who’s had an accidental drug overdose. If you overheard her joking at a coffee counter, asking for a unicorn drawn in her cappuccino foam, you wouldn’t guess that years of relentless pain have left her clinically depressed.
“I can’t even clean the dishes in my sink. I can’t even put the toilet paper rolls in the bathroom, or make my bed in the morning, because I just don’t want to do anything,” she said, describing how she feels on her worst days. “And that’s so unlike me.”
Morse says she has always had a “get-up-and-go” personality. She worked for years as a hospital marketing representative and raised two daughters who are now in college. With her own kids out of the house, she volunteers with local foster children at a Santa Cruz County non-profit organization. In her free time, Morse’s ideal day would be filled with mountain biking, paddleboarding, and canyoning, followed by beach volleyball at sunset.
But none of this is possible without Morse’s pain medications, and often, they don’t do the trick.
She takes two tablets of the narcotic Vicodin, two to three times a day, along with a nerve pain medication, Topamax, that makes her face twitch involuntarily. That’s in addition to roughly bimonthly injections of a nerve blocker — a medication that numbs the nerves in her neck to prevent the pain in her arm. She recently upped her Cymbalta prescription to treat her depression as well as her nerve pain. Her medications put her in a fog, make her drowsy, sap her motivation and disrupt her digestion. On bad days, the pain keeps her from her favorite activities, and from her work with foster children.
“How can I be there for them when I’m not there myself?” Morse says.
Tawfik envisions a better future for Morse and patients to follow.
“The end goal would be that a patient comes into clinic, they get a scan, and we can see that their microglia are activated,” Tawfik says. “Then we can say, ‘You’re appropriate for treatment with this drug that modulates those cells.’”
Tawfik said this ideal vision of tomorrow is a long way off, and her preliminary results are just that: preliminary. The road from mouse model to medical marvel is pitted with many challenges.
Other researchers are pursuing similar goals. Linda Watkins helped usher a glia-targeting drug into human clinical trials in March of 2018. The drug aims to interrupt the signals that glial cells use to turn up pain in patients with arthritis. It’s already worked in rats, dogs and horses, and Watkins hopes it will prove effective in people.
“There’s been 25 years of research. It’s really time to get past the FDA,” Watkins says.
Tawfik agrees. She hopes one day to offer Stephanie Morse permanent escape from the pain that has held her hostage since 2008 — a pain so severe that Morse has almost forgotten what life was like without it.
Imagine if Tawfik held the power to just switch that pain off.
© 2018 Nicoletta Lanese / UC Santa Cruz Science Communication Program

Nicoletta Lanese
Author
B.S. (neuroscience) and B.F.A. (dance) University of Florida
Internships: Stanford University School of Medicine news office, Monterey Herald, San Jose Mercury News
In second grade, I wrote construction-paper novels about the misadventures of feisty heroines. I dreamed of becoming a professional writer—until neuroscience beguiled my mind and dance captured my heart.
I chased three careers in college: scientist, dancer, and writer. Flushed from morning dance classes, I dashed to the lab to record freshmen’s brain waves each afternoon. Then I raced back to the studio, where I translated movement disorders into dance steps before hustling home to blog by moonlight.
Everything changed when a professor assigned me to summarize a complex cognitive psychology study. Marveling at the clarity of my explanation, my professor remarked that I would make a good science writer. I now pursue my dream job in science communication.

Sylvia Heredia
Illustrator
B.A. (biology) Universidad de los Andes
M.Rs. (environmental sciences) University of Edinburgh
Ph.D. (botany and plant sciences) University of California, Riverside
Internships: Maimon Lab, The Rockefeller University (New York, New York), Goldbogen Lab, Hopkins Marine Station (Pacific Grove, California), Gregory Crutsinger, Scholar Farms
I vividly remember running in the damp backyard of my childhood home in Bogotá-Colombia, chasing my sister while holding a small silky green dotted frog in my hands. Nature has always been a source of playful beauty and inspiration for me. As a biologist, I love the moments when I discover something new through close observation, when details overcome the generalities. As a science illustrator, my instinct is to draw the insight to be able to capture, understand and communicate. My work capitalizes on all the inspiration and fun I had with those beautiful green dotted frogs… and hopefully in the process, makes amends with my sister!

Brett Bell
Illustrator
B.A. (environmental studies and biology) University of California, Santa Cruz
Internships: Ventana Wilderness Alliance (Santa Cruz, CA), Stephen McCabe, UC Santa Cruz Arboretum (Santa Cruz, CA), UC Santa Cruz Fort Ord Natural Reserve (Marina, CA)
Brett’s passion for visual storytelling and illustrating science comes from a simple curiosity for the natural world and a love of making things. He hopes his work will allow broader audiences to access scientific concepts and be inspired to explore the natural world around them.