The fly, the mouse, and the monastery: how one mountaintop retreat gave way to a new scientific field
As scientists around the globe started noticing the same phenomenon cropping up in their labs, they joined forces to study how animals withstand infection—independently of pathogen-killing—in a process called “disease tolerance,” Annie Melchor reports. Illustration by Gillian Marie.

Photo: Annie Melchor
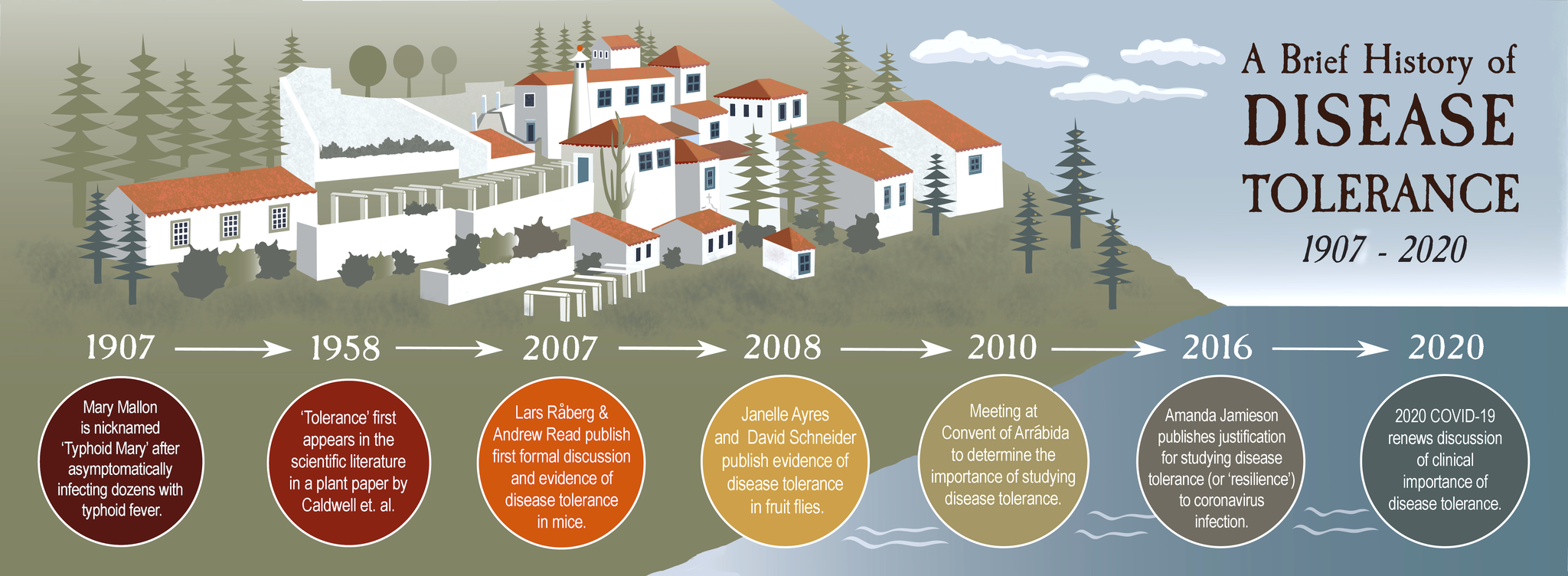
It was 2010 and recent PhD graduate Janelle Ayres had just arrived at the Convent of Arrábida—a sixteenth century Franciscan monastery outside Lisbon, Portugal—for a very special scientific meeting. Perched on a wooded mountainside, the monastery’s red-tiled roofs peeped out of the surrounding forest, overlooking the Atlantic Ocean. Although she was one of the only junior scientists in attendance, Ayres was uniquely prepared for the meeting, which had been called to discuss a new concept called “disease tolerance” that was the backbone of her dissertation research. She didn’t know then that the decisions made at that meeting would still be shaping her research more than a decade later.
When people first learn about the immune system, they often imagine a castle under siege. Thick walls form a protective barrier around the castle, and anyone trying to scale those walls is met with flaming arrows and boiling oil raining from the ramparts. Like the castle’s walls, skin and moats of mucus keep most microbial invaders out of our bodies. And for anything that tries to slip past those defenses, the immune system has a whole arsenal of weapons to trap, banish, and destroy them.
But anyone who actually lived in a medieval castle knew that offensive weaponry isn’t the only thing you need to withstand a siege. Farmers have to grow and harvest food to sustain the soldiers. Craftsmen repair breaches in the castle walls and physicians heal injured warriors. Despite the chaos outside, mothers bear and raise children to become the next generation of soldiers and farmers and physicians. Even though none of these things are defensive military strategies, they’re still essential for keeping the fortress running both during and after the siege. This is the principle behind disease tolerance, which “enable[s] the body to adapt to the presence of the pathogen,” without trying to eliminate it, said Ayres, who now runs her own lab at the Salk Institute.
Plant biologists and farmers have understood this phenomenon for over a century. When infected or drought-stricken plants are able to survive stressful conditions while still being fruitful, they are said to be “tolerant,” and many plants are selectively bred for these traits.
But what about disease tolerance in animals? If two animals were infected with the same amount of the same pathogen, could one tolerate it better than the other? Until the meeting at Arrábida, no one had really talked about it—although understanding why some people get sick from infections while others don’t has had doctors scratching their heads from Typhoid Mary to today’s asymptomatic Covid-19 super-spreaders.
The scientists gathering at the monastery were finding experimental evidence that animals do, in fact, have different ways of tolerating disease. If this were true, it would mean the discovery of a historically overlooked branch of animal immunity.
The convent’s strikingly white-washed walls and cobblestone walkways, punctuated with Mediterranean greenery, had borne witness to nearly 500 years of history. It was now entirely possible that it was about to watch history in the making—by having front row seats to the birth of a new scientific field.
“The most obvious thing in the world”
Andrew Read is an evolutionary ecologist at Penn State University with a quick wit and a thick New Zealand accent. Although he mainly studied pathogen evolution, Read had long harbored a fascination with plant tolerance.
Think about the grass in your lawn, he said. “It gets massive amounts of it chopped off every week—and it still does fine.” Instead of dissolving into a heap of grassy, green carnage, a freshly mowed lawn just…grows back.
In 2007, a scientist in Read’s lab named Lars Råberg wanted to test the possibility of disease tolerance in mice with a simple experiment: Råberg would infect mice from different genetic backgrounds with a parasite to give them malaria. Then, he’d measure the relationship between how many parasites were in the mice and how sick they got. If some mice got less sick than others, despite having the same total number of parasites as the sicker mice, it would indicate they were tolerating the infection better.
And this was exactly what he saw. Some strains of mice were more tolerant of malaria than others—staying healthier despite the parasites living inside them—making it the first documented evidence we know of disease tolerance in animals.
Even though Read was “completely unsurprised” by the results, they were published in the prestigious journal Science. “To this day, I’m still staggered that we happened to be the first to do that,” he said. Because to him, “it’s the most obvious thing in the world.”
“You need to ask the fruit fly what’s important”
On the other side of the country, Stanford immunologist David Schneider and Janelle Ayres, then a PhD student in his lab, were making similar discoveries with very different tools.
“But what if immunity is way more than just these pathways?”
Ayres and Schneider had been studying immunity in Drosophila melanogaster—the common fruit fly. Historically, fruit fly researchers had focused on studying only one or two strategies or “pathways” the fly uses for immune defense. “But what if immunity is way more than just these pathways?” they wondered. To answer this, said Schneider, you need to ask the fruit fly what’s important.
Ayres gave an infection to fruit flies with mutations in random genes that made it so those genes were no longer able to carry out their normal jobs. Some of the mutated genes were known to fight pathogens, but for most of them, their role in infection was completely unknown.
“What you would have expected from people’s discussions of fly immunity before,” said Schneider, “is that fly immunity was all about killing microbes.” So you’d expect that the flies dying the fastest from the infection were the ones with mutations in immune defense genes. (Going back to our castle analogy, these flies were essentially the ones with broken arrows or lukewarm oil—some kind of defect in their offensive weaponry that made it so they couldn’t fight the infection and were overrun with bacteria.)
But this wasn’t the whole story.
Some of the flies that died quickly had “a perfectly normal number of microbes,” said Schneider, meaning that although they had no problem keeping the bacteria in check, their mutations kept them from being able to tolerate the infection. Ayres and Schneider published their findings about six months after Read’s Science paper.
Avengers Assemble
Miguel Soares is an immunologist at the Gulbenkian Institute in Portugal who, like Read, was also studying malaria in the mid-2000’s. When he stumbled upon Råberg’s paper, he was initially annoyed. Unlike most Science papers that are packed to the brim with graphs and charts, this one had hardly any data.
“How can these guys publish this? There’s nothing here!” he remembers thinking.
But then he went back and read it. “And it’s a really important paper. It’s not simple.” Soares hadn’t realized that his own malaria experiments had also been pointing towards disease tolerance. Now he had the vocabulary to describe what he had been seeing—and other researchers to talk to about it, including Yale immunologist, Ruslan Medzhitov.
When he read the papers from Schneider and Read’s labs, Medzhitov thought the idea that there could be drastically different outcomes of infectious disease, regardless of the number of pathogens was a “striking revelation,” and he quickly began thinking about how it could open up brand new lines of research and answer new questions within infection biology.
Meeting of the Minds
In 2010, Soares organized the meeting at the Convent of Arrábida. Ayres, Schneider, Råberg, and Medzhitov were there, along with a mix of other scientists: hardcore immunologists, plant biologists, evolutionary ecologists and a few researchers from Soares’s own Gulbenkian Institute. In total, there were 26 attendees—many of them meeting each other in person for the first time.
Soares said the driving force of the meeting was to explore to what extent disease tolerance was true, so that’s what they did. Because it was at a monastery, there wasn’t much else to do besides talk science. Lots and lots of science.
“We had talks all day, and then we had dinner and wine hour where we just talked science the entire time—just bantering back and forth on what disease tolerance is,” said Ayres. “It was amazing.”
Schneider remembers something else that helped things go smoothly: “After the support staff would go to sleep, they’d leave out a bunch of bottles of this Portuguese Moscato—which was like fruit juice that packed a real punch,” he said. “It really opened up the conversation,” he grinned, eyes twinkling.

The team who met at the Convent of Arrábida in 2010 to discuss disease tolerance. Photo property of Miguel Soares.
After two science and Moscato-filled days, the group came to a consensus about disease tolerance.
“This is big biology,” said Soares, recounting Medzhitov and Alexander (“Sasha”) Rudensky’s reaction in their Russian accents.
The meeting attendees determined that disease tolerance in animals was a fundamentally important arm of biology shared between organisms as diverse as plants, insects, and mammals. They also made another important decision—to collaborate in building the new field instead of fighting amongst themselves about who had discovered disease tolerance first. Everyone had their own niche, said Schneider, so there was plenty of room to grow.
Disease tolerance in the time of coronavirus
Flash forward to 2021: a lot has changed. Because of Covid-19, discussions about disease tolerance have leapt from the halls of Arrábida to dinner tables around the world, even if people don’t know that’s what they’re talking about. “How can I be infected if I don’t even know I’m sick?” and “How can doctors help patients without knowing how to fight SARS-CoV-2?” are common questions.
But they’re not new for everyone. Brown University immunologist Amanda Jamieson first encountered disease tolerance when she worked in Medzhitov’s lab, and she has continued to study disease tolerance in the lungs.
In 2016, she published a paper about the importance of studying tolerance (she called it “host resilience”) to coronaviruses, many of which cause severe inflammation and subsequent damage in the lungs. “The lung has this really beautiful, fine structure,” she said. She explained that our central airways branch from the trachea into alveolar sacs in the lungs, which are little “balloon-like things that are essential for air exchange—basically essential for keeping you alive.”
When those air sacs get damaged during infection, breathing becomes difficult –sometimes to the point of total respiratory failure and death. Even if someone survives a severe lung infection, the damage to the delicate lung tissue can be permanent, so finding ways to limit damage is critical, especially when fighting a brand-new virus like SARS-CoV-2.
Developing drugs to fight new pathogens takes a long time, and there’s always a risk the pathogen will evolve a way to evade it. But Jamieson said drugs that promote disease tolerance could be applied to a broad range of different pathogens. And, since disease tolerance targets the host, not the pathogen, there’s no pressure for the pathogen to evolve around the drug.
Schneider pointed out that using supplemental oxygen to treat Covid-19 is a perfect example of helping a patient tolerate infection. The oxygen does nothing to kill the virus, but it bolsters your reduced lung capacity and keeps you alive long enough for your body to fight the infection.
From this perspective, it may be tempting to see disease tolerance during infection as the knight in shining armor, riding in on a white horse to save the day.
“We have a huge [to see] disease tolerance as being good,” said Soares. We like being able to see clear divisions between good and evil— “that’s why Star Wars and Lord of the Rings are so successful,” he said. “These guys are bad and these guys are good, and it’s simple.”
But,” he added, “life is never like that.”
Soares went on to explain that even though disease tolerance can be life-saving at an individual level, it can have devastating consequences in a population when some people are less tolerant of a disease than others.
Consider the case of Typhoid Mary, the infamous early twentieth-century cook who asymptomatically carried the strain of Salmonella that causes typhoid fever, and unknowingly infected over 50 people in New York City. Mary had high disease tolerance to the Salmonella, so even though the bacteria were actively replicating inside her and spreading to others, she tolerated it—she didn’t get sick. Her unwitting victims, however, were less tolerant and became very ill. At least three died.
Soares says we don’t even need the Typhoid Mary example anymore because of Covid-19. People with higher disease tolerance may be able to asymptomatically carry the virus, spreading it to others who—for genetic, environmental, or socioeconomic reasons—are less able to withstand the damage caused by SARS-CoV-2 infection and end up getting really sick.
“If we intervene in infectious disease patients by promoting disease tolerance, is that actually bad for the community at the population level?”
Because of this, Ayres warns that population-level health has to be considered before promoting disease tolerance to infections. “If we intervene in infectious disease patients by promoting disease tolerance, is that actually bad for the community at the population level?” she asked. Would this create another Typhoid Mary situation?
Not necessarily. “You can imagine if you promote disease tolerance in a host, you can buy them enough time for their own immune system to kick in and clear the infection,” she said. “Or, you can enter again with antibiotics or antivirals and so you can ultimately clear it then.” The important thing is ensuring the pathogen is fully cleared so it can’t asymptomatically spread to others.
By working to understand and (when appropriate) promote disease tolerance during infections, Ayres said we could be better equipped for when the next pandemic hits—keeping patients alive while we’re waiting for effective antivirals and for vaccines to be developed.
Looking to the future
Despite the clinical importance of understanding disease tolerance and the enthusiasm within the little field, many of the early organizers say it hasn’t caught on as quickly as they had hoped.
“It’s interesting that despite the fact that it’s such a natural and powerful way to look at infectious disease and infection biology, [disease tolerance] actually did not explode the way you might one might expect.”
“It’s interesting that despite the fact that it’s such a natural and powerful way to look at infectious disease and infection biology,” said Medzhitov, disease tolerance “actually did not explode the way you might one might expect.”
But perhaps, you would expect it to take some time. For decades, immunology research has focused on the amazing weaponry our bodies use to fight pathogens and cancer cells. And while this research has led to some incredible medical advances, it clearly wasn’t the whole picture. New ideas that challenge existing paradigms take time to accept. But the fact remains that the field is here to stay.
Although the growth may feel slow, the ideas discussed in Arrábida are spreading. In the last few years, groups from Singapore, Belgium, Spain, Nigeria, Switzerland, the United Kingdom, Germany, as well as many other countries have published papers on disease tolerance in animals, indicating the growing international interest. Perhaps it’s too early to plan another meeting just yet—but it looks like we’re going to need a bigger monastery.
© 2021 Annie Melchor / UC Santa Cruz Science Communication Program

Annie Melchor
Author
Ph.D (experimental pathology) University of Virginia
Internships: Stanford Linear Accelerator Laboratory Press Office, Monterey Herald, & Symmetry Magazine
At first, my microbiology major in college didn’t make sense to anyone, including myself. I was a hopeless romantic who loved literature, languages, history, and theater. But I found that the microbial world was also full of drama. And when I joined a lab, I fell in love with the scientific method. It felt like playing a mind game with the universe: If I thought of the right way to interrogate my hypothesis, I could test it, and learn something no one else in the world knew.
Later, as a Ph.D. student in a brand-new lab, I developed a scrappy drive to do whatever it took to answer our questions. Now, as a science writer, I use the starry eyes and hard-boiled skepticism I cultivated during my scientific training to tell accurate and compelling science stories.

Gillian Marie
Illustrator
B.A. (Fine Arts/Journalism double major) Otis College of Art and Design
My love of nature and art was born at our kitchen table in my childhood home in the woods of upstate New York. I didn’t know it then, but the simple act of gathering flowers and pressing them between the pages of the book into bookmarks was planting a seed in my psyche. It instilled a love of nature, color, and art that my mom nurtured endlessly. She filled our days with bird-watching, drawing, and exploring the frozen pond behind our house aptly named Sugar Pond. The kitchen became a casual art studio, a science lab, a magical space full of acorns, leaves, pencils, coloring books, twigs, dried flowers, color, and light.
I went on to study art in college, became an art teacher and then a graphic designer. Now, after raising my own children, I’m returning to school to combine my passions into a new exciting field. My studio these days is in Boulder, Colorado and is still a magical space full of acorns, leaves, pencils, twigs, dried flowers, color, and light.